Organic Chemisty
F. López Ortiz research group
University of Almería
| HOME | ABOUT FLO | RESEARCH | PUBLICATIONS | GROUP |
Research summary
Our research is focused on metalation reactions directed by phosphorus-containing functional groups as a tool for developing new methodology with applications in asymmetric synthesis, coordination chemistry, and catalysis. The rational design of the synthetic work is supported by mechanistic studies of the transformations through multinuclear magnetic resonance. Recently, we have extended the use of NMR techniques, especially HRMAS, in combination with statistical methods to metabolic studies of vegetables produced under greenhouses. Main specific research interests:
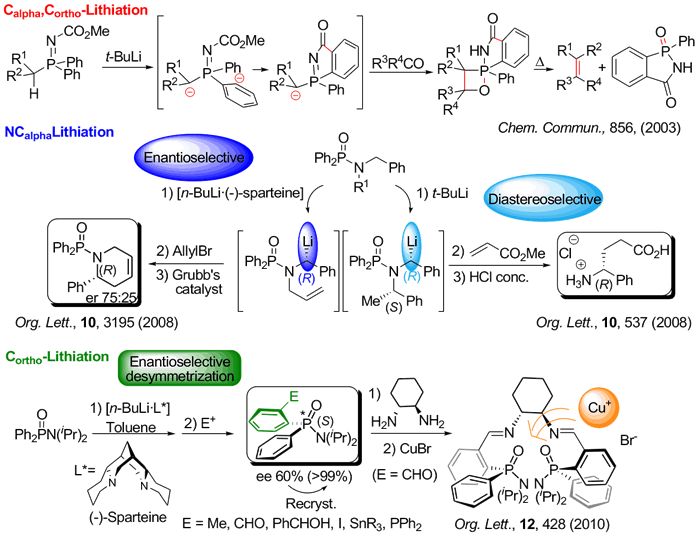
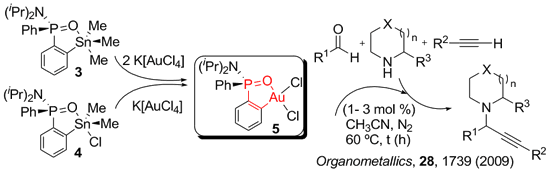
Selective Calpha, Cortho, and NCalpha lithiation of organophosphorus compounds containing the Ph2P=X moiety followed by electrophilic quench affords new functionalized derivatives that can be transformed into a variety of highly added value molecules.
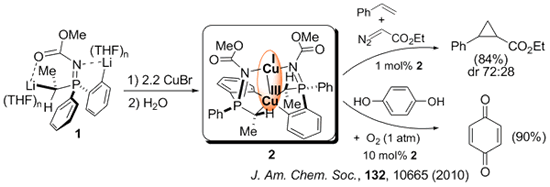
The reaction of the Calpha,Cortho dilithiated phosphazene 1 with CuBr provided the first example of a binuclear mixed valence [CuI(N2)/CuIII(C4)] complex 2 showing a metal-metal bond. This complex catalyzed prototypal oxidation and cyclopropanation reactions involving Cu(I) and/or Cu(III) species.
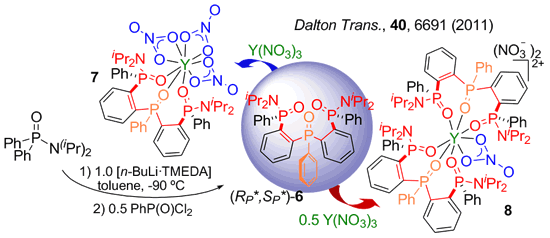
The first step of our synthetic work consist of a lithiation directed by a Ph2P=X group. NMR monitoring the lithiation step in combination with DFT computational studies allows for a rational design of the synthesis. We have developed a NMR methodology based on 31P detection to get structural information through correlations with non conventional nuclei. Of particular interest for us are 7Li and 89Y. Representative examples of the species identified in solution are shown below.
HRMAS NMR in combination with unsupervised multivariate analysis have been used to study metabolite changes associated to tomato ripening and tissue differentiation. Flesh, peel and seeds from mature red fruits were separately analyzed. |
||||||